Liebe Kunden und Partner,
wir müssen Ihnen leider mitteilen, dass die Spindiag GmbH aufgrund einer Insolvenz zum 1.10.2023 ihren operativen Betrieb einstellt. Dieser Schritt fällt uns sehr schwer, denn wir haben uns mit Leidenschaft und Engagement für die Verbesserung der Infektionsdiagnostik eingesetzt. Wir sind stolz, dass wir mit unserem Rhonda System einen wichtigen Beitrag zur Bewältigung der COVID-19 Pandemie leisten konnten. Unser Rhonda System hat es ermöglicht, Patienten schnell und zuverlässig zu testen und so die Ausbreitung von Infektionen zu verhindern.
Wir bedanken uns von ganzem Herzen bei Ihnen für Ihr Vertrauen und Ihre Unterstützung. Wir sind dankbar für die vielen spannenden Projekte und die wertvollen Erfahrungen, die wir mit Ihnen teilen durften. Wir wünschen Ihnen für die Zukunft alles erdenklich Gute.
Ihr Spindiag-Team
Dear customers and partners,
we regret to inform you that Spindiag GmbH will cease its operational activities as of October 1, 2023, due to insolvency. This step is exceedingly difficult for us, as we have dedicated ourselves with passion and commitment to improving infection diagnostics. We are proud that we were able to make an important contribution to coping with the COVID-19 pandemic with our Rhonda system. Our Rhonda system has enabled us to test patients quickly and reliably and thus prevent the spread of infections.
We thank you sincerely for your trust and support. We are grateful for the many exciting projects and the valuable experiences that we were able to share with you. We wish you all the best for the future.
Your Spindiag team
The challenge
Infectious diseases
Waiting for lab results

Risk of infection
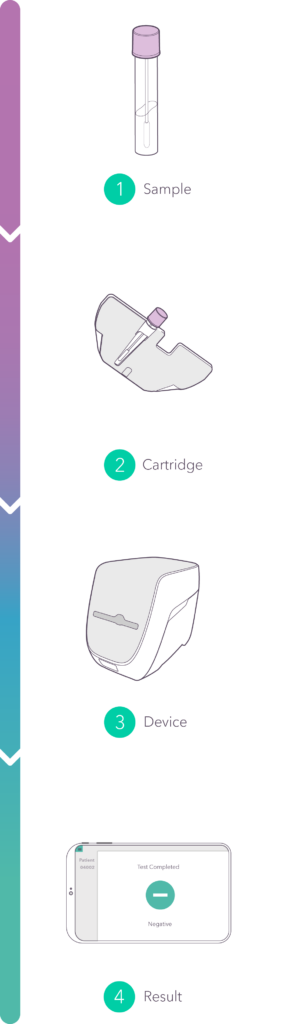
The point-of-care solution to control infectious diseases…
providing results for close to 60 patients per device right at the patient admission every day.
Seven days a week.
Safe
Simple
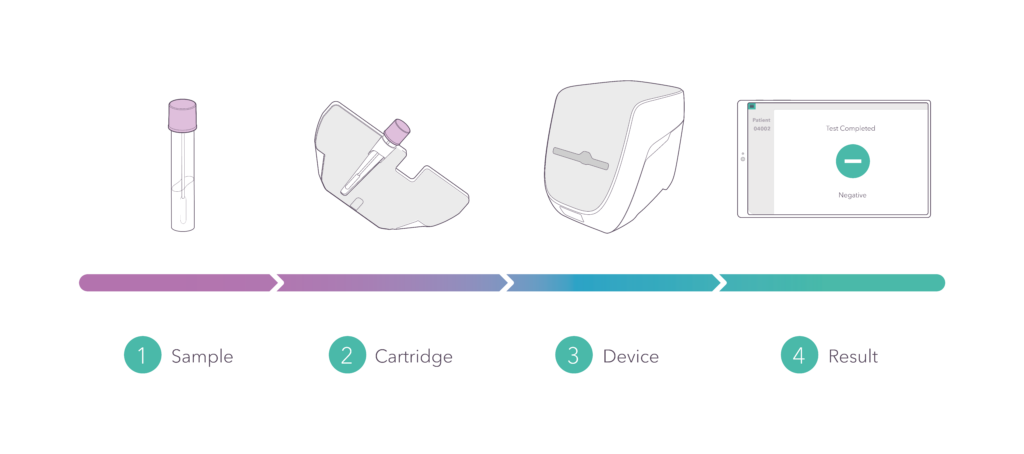
No sample prep, no pipetting, no additional reagents: from swab to decision in well under one hour. Decide on the spot: quarantine or not.
Efficient
Centrifugal microfluidic technology
Rhonda disk
Insert a standard COPAN eSwab® . Follow your usual routines.
Ultrafast and sensitive PCR for fast and reliable results.
Affordable even for screening applications.

Rhonda player
Three buttons, no touch. Simple is back.
Two analyses for the same pathogens can be analyzed in one run.
One player for numerous applications. Introducing the Rhonda platform.

„Die Rhonda waren auf unseren Intensivstationen ein echter Durchbruch, nicht zuletzt, weil die Geräte so einfach und bedienerfreundlich sind. Anfangs hatten Viele Bedenken in Bezug auf ein Ansteckungsrisiko – das legte sich schnell, als klar wurde, wie unkompliziert die Bedienung des Systems ist.”
„Rhonda ist im Klinikalltag nicht mehr wegzudenken und bietet in verschiedenen Bereichen einen Mehrwert. Zum Beispiel für eine anstehende Verlegung von Patienten in Reha- und Nachsorgekliniken ist ein tagesaktuelles, schnelles SARS-CoV-2 PCR-Ergebnis oft entscheidend. Mit Rhonda ist dank kurzer Testzeit nach spätestens 1,5 Stunden klar, ob der Patient in die Reha verlegt werden kann oder nicht. Auch im Aufnahmemanagement und der Bettenplatz-Zuordnung können wir jetzt viel schneller Entscheidungen treffen – zuvor mussten häufig Betten umgeschoben werden, was dank Rhonda entfällt und die Arbeit für die Pflege extrem erleichtert.”
“Die Analysen sind schnell, valide und anwenderfreundlich”
“Kritisch kranke Notfallpatienten mit unklarem Infektstatus wurden unmittelbar mit Beginn der Versorgung in der Notaufnahme oder im OP getestet. Mit der sehr schnellen und validen Verfügbarkeit der Ergebnisse konnten dann die hygienischen Vorsichtmaßnahmen prompt getroffen werden.”
“Dank der schnellen Ergebnisse von Rhonda konnten wir Dutzende OPs durchführen, die ansonsten wegen nicht vorliegender Laborergebnisse ausgefallen wären.“
“I think this system particularly makes sense in intermediate care wards. From my own experience, if a patient is admitted to the hospital and the history indicates previous MRSA or 4MRGN, it’s useful to know within half an hour whether I need to isolate the patient and initiate further steps.”
“In my experience, the system is very useful and very helpful, especially with the functions I saw. It saves a lot of effort and time, in terms of days and beds – just what Spindiag designed it for. The system is a really good idea! I didn’t know this was technically possible. Awesome!”
“Spindiag´s system makes a lot of sense. I witness it myself: In the hospital, two patients are kept together, and late in the process it turns out that one patient has a pathogen. With such a system, you could recognise much faster: You need to isolate the patient.”
“The system is very useful! Since we receive many patients from external hospitals, we must isolate them on arrival. This costs a lot of time and money for everyone. In our case, it is meaningful to get the test result within half an hour.”
“This system makes a lot of sense and saves a lot of work, since you find out in only 30 minutes if the patient needs to be isolated or not!”
“Die Rhonda player sind bei uns ständig im Einsatz und bereichern den Alltag unserer Mitarbeiter und Patienten. Für uns sind die player ein riesen Gewinn, da wir die Bettennutzungszeit durch den Einsatz von Rhonda signifikant reduzieren können. Das betrifft im Besonderen unsere ZNA.”
Who we are
Founded in 2016, Spindiag is a spin-off from the renowned research institute Hahn-Schickard. From the vision of seven scientists, a young innovative medical technology start-up was formed, consisting of a highly motivated continuously growing team of more than 100 employees today. Spindiag comprises a team with valuable know-how from different fields in science, research & development, production, marketing, quality, sales, and services supported by external advisors.
We aim to arm the world with an effective tool against epidemic and pandemic infectious diseases: for this purpose, we introduced the point-of-care testing system Rhonda, quickly delivering reliable in-vitro diagnostic PCR results in well under one hour. We envision, that patients will be safer thanks to Rhonda, the new gold standard for routine infection control. Providing a safe, simple, and efficient solution to healthcare workers, to perform routine infection control.
In doing so, we follow our mission every day to improve world health by simply providing diagnostic results.
In 2020, we launched a point-of-care PCR rapid test for the detection of SARS-CoV-2. The video illustrates the pandemic situation that threatened the world, and the solution that Spindiag is offering. It showcases the complete workflow from sample collection to result.
Enjoy our video here!
Contact
Germany
Spindiag Head Office
Phone: +49 761 600 49 600
E-Mail: info@spindiag.com
Spindiag GmbH
Engesserstrasse 4a
79108 Freiburg im Breisgau
Austria
Spindiag
Phone: +49 761 600 49 600
E-Mail: info@spindiag.com